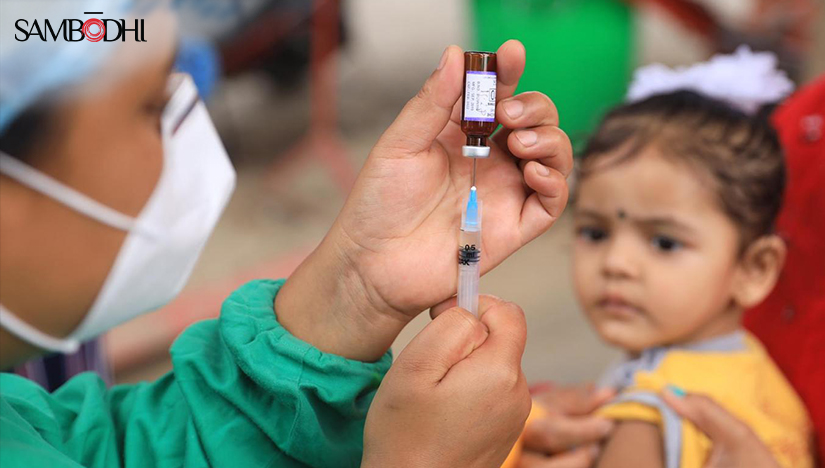
What do we know about Tuberculosis (TB) to date?
- Its molecular evidence dates to more than 17,000 years.
- WHO has declared it to be the 13th leading cause of death.
- 1.5 million people died from TB in 2020, making it the second leading infectious killer after COVID-19
- Thirty nations have the highest TB burden, making up 86% of all new TB cases in 2020.
- Eight nations, including India, which is at the top of the list, followed by China, Indonesia, the Philippines, Pakistan, Nigeria, Bangladesh, and South Africa, are responsible for two-thirds of the total burden.

These statistics suggest the deadly nature of the disease, but what have we done to curb its effects so far?
In the Indian context, a nationwide program called National Tuberculosis Elimination Program (NTEP) has been working since 1992 to combat the burden of such a debilitating disease. Notable advancements in tuberculosis prevention, care, and control over the decades have led to more than 80 million people being screened for TB and 15 million patients being successfully treated, having saved millions of lives.
Yet, India continues to contribute the largest number of TB patients and TB-related deaths worldwide.
According to the TB India report 2022, 2021 witnessed a 19% increase in TB patients’ notifications from the previous year.
Bacille Calmette Guerin (BCG), developed in 1921, is the only vaccine used in practice for the last 100 years. Today, billions of people have received BCG, making it the most widely used vaccine worldwide. Although BCG has proved effective against disseminated disease in young children, it has variable efficacy against lung TB, particularly in adults.
It is found that the countries with the highest TB incidence have the lowest levels of protection against TB. Therefore, it is indicated that BCG prevents approximately only 5% of all potentially vaccine-preventable deaths due to TB.
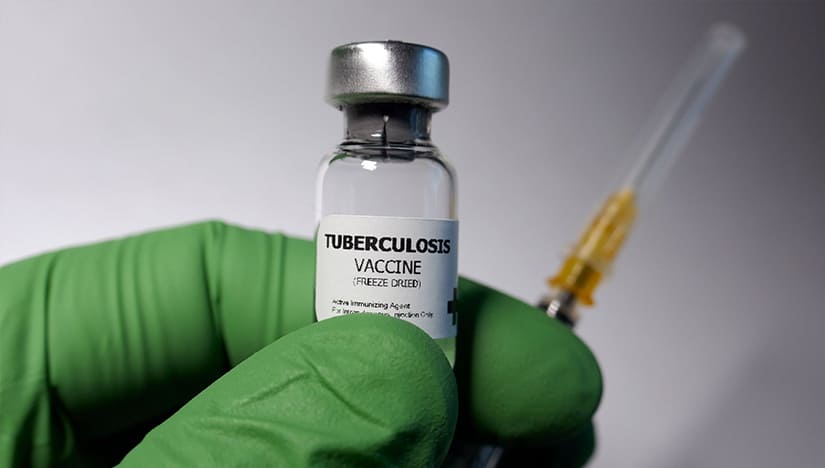
Having known these limitations, several clinical trials have been in the works in the last decade. With more than ten vaccine candidates in different phases of clinical trials, different vaccine technologies are being tested. However, this process needs to be optimized and will require a robust system before rolling out the vaccines in a country as populated as India.
Having said that, this process has not been obstacle free. It has been challenging to develop an anti – TB vaccine primarily for two reasons. Firstly, the immune response generated by BCG is so variable that it is difficult to put the finger on the response that would protect a person from TB. Secondly, little is known about the animal model that best predicts how a vaccine will perform in humans.
Out of the $915 million invested in TB-related research in 2020—or less than half the $2 billion target indicated by the World Health Organization (WHO)—only 13%, or less than $120 million, went towards developing vaccines.
That means that it would take about 500 years of effort to develop a TB vaccine to receive the same amount of funding that COVID-19 received in one year. The COVID-19 pandemic taught us that focused & concentrated efforts with a large-scale investment have the potential to turn the tide. The result included several successful vaccines that have halted the progress of the severe viral disease.
Despite TB being one of the oldest diseases that continues to plague many worldwide, its vaccine developments need health infrastructure, critical evidence-based thinking, and large-scale investment to eliminate it entirely. With an estimated 10.6 million people falling ill to TB in 2021, not to mention the loss to the family in terms of loss of earning potential, we still are running behind in developing successful vaccine candidates. This dire situation demands immediate attention and collaborative partnerships with multi-sectoral involvement.
Roll out of any new effective and safe anti-TB vaccines will be extremely useful, especially in countries with higher incidences and prevalence of TB, such as India. A more consistently effective vaccine than BCG in adolescents and adults is needed to achieve the ‘End TB Strategy’ set by the WHO. More than three effective vaccines were developed for COVID-19 in a short period. Such large-scale research investments can help develop vaccines for TB to contain the accelerating spread of multi-drug-resistant tuberculosis, especially in India.
Based on the lessons learnt from the COVID-19 pandemic, we must ensure and replicate the successes achieved, especially in vaccine development and prepare to address the challenges faced in ensuring vaccine equity. A global coalition reinforced with much-needed political commitment backed by massive investments in research and development of the Tb vaccine can save millions of lives. With the deadline for the elimination of TB approaching, the time to invest in TB vaccines is now. Increased investments can be a turning point and alleviate the suffering and deaths caused to millions due to TB.

Prachi Pravin Karkhanis – Senior Manager, Sambodhi